Epigenetic and Transcriptional Regulation of Central Tolerance
A critical mechanism for the maintenance of immune tolerance is the process of thymic negative selection (central tolerance), whereby autoreactive T cells are deleted in the thymus. Central tolerance is carried out by the medullary thymic epithelial cell (mTEC) which has the remarkable ability express thousands of self-antigens in the context of the major histocompatibility complex (MHC) to developing T cells. Deletion of autoreactive T cell clones occurs, if there is a strong interaction between the T cell receptor (TCR) on the developing T cell and the self-antigen expressed by the mTEC. Consequently, defects in the expression of self-antigens by mTECs has been linked to autoimmunity secondary to the failure to delete self-reactive T cells. As mTECs play a critical role in central tolerance, expanding our knowledge of both mTEC development and mTEC self-antigen expression will allow a more complete understanding of immune tolerance. The lab currently has several ongoing projects studying the importance of specific proteins involved in epigenetic and transcriptional regulation in mTECs, utilizing a variety of conditional knockout mice.
Epigenetic regulation of CD4+ T helper 17 (Th17) Differentiation by ATF7ip
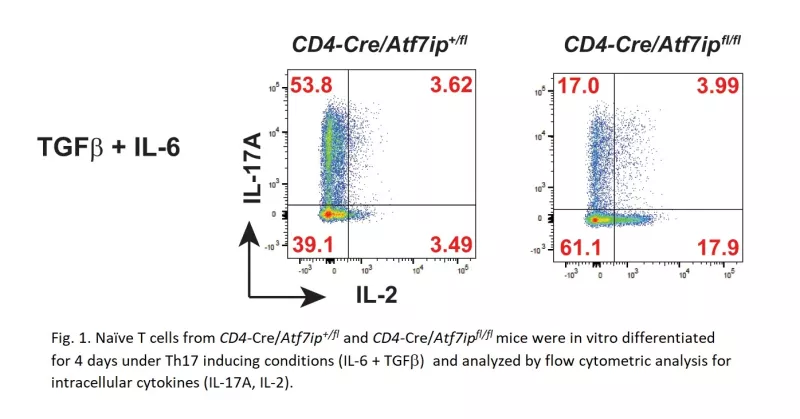
One immune cell type that is important in the pathogenesis of autoimmune disease is the effector CD4+ T helper 17 cell (Th17). Identifying molecular pathways that are essential for Th17 differentiation may be crucial for designing future therapies. Utilizing a novel conditional knockout mouse model for the activating transcription factor 7 interacting protein (ATF7ip), we have identified ATF7ip as a protein critical for Th17 differentiation (Figure 1).
ATF7ip is an epigenetic regulator involved in gene repression through formation of the repressive H3K9me3 mark. Based on ATF7ip's known function in gene repression, we hypothesized that ATF7ip is important in repressing a gene(s) that negatively regulate Th17 differentiation. Global gene expression analysis revealed that ATF7ip is required to silence interleukin 2 (IL-2) in T cells and the deletion of ATF7ip in T cells caused the aberrant overexpression of IL-2 with T cell receptor (TCR) stimulation. Furthermore, H3K9me3 ChIP-seq showed that T cell specific deletion of ATF7ip attenuated H3K9me3 deposition at the Il2-Il21 intergenic region. IL-2 is a known inhibitor of Th17 differentiation, thus IL-2 overproduction is potentially a key mechanism for the defect in Th17 differentiation seen in ATF7ip deficient T cells. Ongoing projects in the lab aim to further define the molecular mechanism of ATF7ip function in T cells and to study the effect of ATF7ip deletion in other immune cell types.
Are you interested in joining the Waterfield Lab? Postdocs and Rotation Students are welcome to apply. Please send your CV to [email protected]